Biomed Industries Advances NA-931 Into Two Global Phase 3 Trials Alone and with Semaglutide and Tirzepatide for Obesity
Biomed Industries to Advance NA-931 Into Two Global Phase 3 Trials as Monotherapy and in Combination With Semaglutide and Tirzepatide for Obesity
Advancing NA-931 into two Phase 3 programs as monotherapy and in combination with semaglutide and tirzepatide underscores our strategy to deliver durable weight loss with lean mass preservation.”
SAN JOSE, CA, UNITED STATES, January 6, 2026 /EINPresswire.com/ -- — Biomed Industries, Inc. (“Biomed”), a clinical-stage biopharmaceutical company developing innovative therapies for metabolic and neurodegenerative diseases, today announced plans to initiate two global Phase 3 clinical programs evaluating NA-931 (120 mg orally once daily) as monotherapy and in combination with established GLP-1–based therapies for the treatment of obesity.— Dr. Lloyd L. Tran, CEO of Biomed
NA-931 is a first-in-class, orally administered small-molecule quadruple receptor agonist targeting IGF-1, GLP-1, GIP, and glucagon receptors, designed to address obesity through integrated neuroendocrine and metabolic mechanisms.
SCIENTIFIC RATIONALE FOR COMBINATION THERAPY:
NA-931 is designed to simultaneously modulate appetite regulation, energy expenditure, insulin sensitivity, and IGF-1–mediated preservation of lean mass. Biomed believes that combining NA-931 with GLP-1–based therapies may enhance the durability of weight loss while addressing limitations associated with single-pathway pharmacotherapy.
PHASE 3 PROGRAM OVERVIEW
1. PHASE 3 OF BIOCOMBO-1: NA-931 Alone and in Combination With Oral Semaglutide
BIOCOMBO-1 is a planned 68-week, randomized, double-blind, placebo-controlled, global Phase 3 study evaluating:
• NA-931 120 mg once daily (monotherapy)
• NA-931 120 mg once daily in combination with oral semaglutide 25 mg once daily
The study is expected to enroll approximately 366 non-diabetic adults with overweight or obesity (BMI ≥30 kg/m², or ≥27 kg/m² with at least one obesity-related comorbidity).
Coprimary endpoints at Week 48 include percent change in body weight from baseline and the proportion of participants achieving ≥5% weight loss. Key secondary endpoints include higher weight-loss thresholds, changes in BMI and waist circumference, and change in IWQOL-Lite-CT Physical Function score.
2. PHASE 3 OF BIOCOMBO-2: NA-931 Alone and in Combination With Injectable Tirzepatide
BIOCOM-2 is a second planned 68-week, randomized, double-blind, placebo-controlled, global Phase 3 study evaluating:
• NA-931 120 mg once daily (monotherapy)
• NA-931 120 mg once daily in combination with injectable tirzepatide 7.5 mg once weekly
The study is expected to enroll approximately 360 non-diabetic adults with overweight or obesity, with endpoints aligned to BIOCOMBO-1 to enable consistent evaluation of weight loss magnitude, durability, body composition, and physical function.
“Advancing NA-931 into two Phase 3 programs as monotherapy and in combination with semaglutide and tirzepatide underscores our strategy to deliver durable weight loss with lean mass preservation.” said Dr. Lloyd L. Tran, Chief Executive Officer of Biomed Industries, Inc.
ABOUT NA-931:
NA-931 is the first-in-class, orally active, small-molecule quadruple receptor agonist that simultaneously targets IGF-1, GLP-1, GIP, and glucagon receptors. This multi-pathway approach restores metabolic balance and induces clinically meaningful weight loss—without muscle loss or severe side effects.
The Phase 2 MAD over 13 week study showed NA-931 demonstrated dose-dependent reductions in mean body weight from baseline, up to 13.8 % at 150 mg daily dosage, or 11.9% % relative to placebo. An exploratory assessment of subjects achieving at least 5% weight loss after 13-week demonstrated that up to 72% of NA-931-treated subjects achieved ≥12% weight loss, compared with 1.9% for placebo. (ClinicalTrials.gov ID NCT06564753).
(Reference: Biomed Industries Presented Phase 2 Results of NA-931: Oral Quadruple Receptor Agonist for Obesity at the Annual Meeting of the European Association for the Study of Diabetes (EASD)- September 15-19, 2026 in Vienna, Austria.)
ABOUT BIOMED INDUSTRIES, INC.
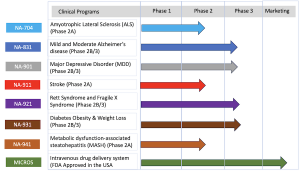
Biomed Industries, Inc. is a clinical-stage biopharmaceutical company focused on developing and commercializing transformative therapies for chronic and complex diseases. The company’s investigational pipeline targets a wide range of unmet medical needs, including:
- Alzheimer’s disease
- Major depressive disorder (MDD)
- Obesity and diabetes
- Metabolic dysfunction-associated steatohepatitis (MASH)
- Stroke and alcohol use disorder
- Rare diseases, including Rett Syndrome and Fragile X
For more information, please visit the website: https://www.biomedind.com
FORWARD LOOKING STATEMENTS
This press release contains forward-looking statements regarding planned clinical trials, development timelines, and potential therapeutic benefits. Actual results may differ materially due to risks and uncertainties. Biomed undertakes no obligation to update forward-looking statements except as required by law.
Michael Willis
Biomed Industries, Inc.
+1 800-824-5135
email us here
Visit us on social media:
LinkedIn
X
Biomed introduction video
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.